Abstract
Background: Coronavirus disease 2019 (COVID-19), a respiratory illness caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic in March 2020. Over 500 million cases of SARS-CoV-2 have been reported worldwide, with over 90 million being in the US. More than 6 million lives, with over 1 million only from the US, have been claimed by the pandemic. Hematopoietic cell transplant (HCT) or chimeric antigen receptor-T (CART) cell therapy recipients have a higher risk of mortality with COVID-19. In this updated analysis, we investigated the outcomes after SARS-CoV-2 infection in HCT/CART recipients.
Methods: We conducted a single-center prospective study, including all (n=196) adult HCT/CART recipients who were diagnosed with COVID-19 at the University of Kansas Medical Center from March 2020 to March 2022. Baseline and disease-related characteristics were ascertained from medical records. Data were analyzed using SPSS version 21 (SPSS Inc, Chicago, IL). Bivariate analyses, using the chi-square and t-test, were performed. Kaplan-Meier and cox regression analyses were conducted.
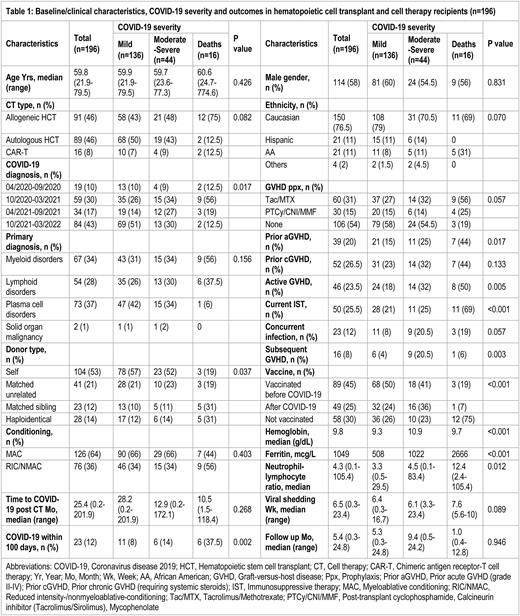
Results: The study included 196 HCT/CART recipients who acquired SARS-CoV-2 infection, including allogeneic HCT (n=91, 46%), autologous HCT (n=89, 46%), and CART (n=16, 8%) recipients. The median age was 59.8 (21.9-79.5) years; 76.5% were Caucasians and 58% were males. The median time since HCT/CART to SARS-CoV-2 infection was 25.4 (0.2-201.9) months, and 12% of patients acquired SARS-CoV-2 within the first 100 days post-HCT/CAR-T. Plasma cell (37%), myeloid (34%), lymphoid (28%) or solid (1%) malignancies were primary diagnoses. Myeloablative conditioning was performed in 64% of patients. Donors were autologous (53%), matched sibling (12%), matched unrelated (21%), and haploidentical (14%). Prior acute graft-versus-host disease (GVHD), prior chronic GVHD, active GVHD, and current immunosuppressive therapy (IST) were noted in 20%, 26.5%, 23.5%, and 25.5% of patients respectively. Concurrent infections were observed in 12% of patients. COVID-19 severity was mild (n=136, 70%), moderate (n=24, 12%), severe (n=20, 10%) or critical/fatal (n=16, 8%). Clinical findings included pneumonia or abnormal chest imaging (31%), hypoxia (19%), intensive care unit admission (9%), and mechanical ventilation (6%). Therapies included remdesivir (31%), convalescent plasma (11%), dexamethasone (15%), monoclonal antibodies (32%), and tocilizumab/baricitinib (5%). At least one dose of SARS-CoV-2 vaccine was reported in 70% of patients (45% pre-COVID, 25% post-COVID) while 3% of patients had received pre-exposure prophylaxis. The median duration of viral shedding (positive SARS-CoV-2 PCR) was 6.5 (0.3-23.4) weeks. After a median follow-up of 5.4 (0.3-24.8) months, the mortality rate was 12% with COVID-specific mortality of 8%. Of allogeneic HCT patients, 18% (n=16) developed subsequent GVHD after COVID-19 requiring systemic steroids and additional immunosuppression. Eighty-four (43%) patients were diagnosed with COVID after Sep 2021 and had the lowest COVID-specific mortality (2% compared to 12.5% in preceding months of the pandemic). Significant predictors of COVID-19 mortality included allogeneic HCT (HR 3.7, 95% CI 1.2-11.6, p=0.023), COVID-19 within 100 days of HCT/CART (HR 4.8, 95% CI 1.7-13.2, p=0.002), COVID-19 diagnosis earlier in the pandemic (HR 5.2, 95% CI 1.1-24.4, p=0.037), prior acute GVHD (HR 3.2, 95% CI 1.2-8.5, p=0.022), active GVHD (HR 3.5, 95% CI 1.3-9.2, p=0.013), and concurrent IST (OR 7.0, 95% CI 2.4-20.1, p<0.001) while prior SARS-CoV-2 vaccination significantly reduced the COVID-19 mortality (HR 0.19, 95% CO 0.05-0.68, p=0.010). In 15 patients with available data, vaccination induced protective anti-spike protein antibody response (>100 U/mL) in 67% of patients with a median titer of 656 (9-688,000) U/mL after completion of the primary vaccination series.
Conclusion: Hematopoietic stem cell transplant and CART cell therapy recipients have a higher risk of morbidity and mortality with SARS-CoV-2 infection. Our findings highlight factors associated with COVID-19 mortality in HCT/CAR-T recipients. In HCT/CART recipients, strict vigilance, infection prevention measures, vaccination prioritization, close monitoring, and early aggressive treatment interventions are suggested for COVID-19 and future emerging infectious diseases.
Disclosures
Abhyankar:Incyte: Consultancy, Research Funding, Speakers Bureau; Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk:Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Orca Bio: Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria; Sana: Honoraria; Novartis: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal